 Unique device identification (UDI) is a "special medical device identification system" established by the U.S. Food and Drug Administration. The implementation of the registration code is to effectively identify medical devices sold and used in the U.S. market, no matter where they are produced. . Once implemented, the NHRIC and NDC labels will be abolished, and all medical devices need to use this new registration code as a logo on the outer packaging of the product. In addition to being visible, UDI must satisfy both plain text and automatic identification and data capture (AIDC). The person in charge of labeling the device must also send the exact information for each product to the "FDA International Specialty Medical Center". The device identification database UDID” enables the public to query and download relevant data (including information from production, distribution to customer usage, etc.) by accessing the database, but the database will not provide device user information.
Unique device identification (UDI) is a "special medical device identification system" established by the U.S. Food and Drug Administration. The implementation of the registration code is to effectively identify medical devices sold and used in the U.S. market, no matter where they are produced. . Once implemented, the NHRIC and NDC labels will be abolished, and all medical devices need to use this new registration code as a logo on the outer packaging of the product. In addition to being visible, UDI must satisfy both plain text and automatic identification and data capture (AIDC). The person in charge of labeling the device must also send the exact information for each product to the "FDA International Specialty Medical Center". The device identification database UDID” enables the public to query and download relevant data (including information from production, distribution to customer usage, etc.) by accessing the database, but the database will not provide device user information.
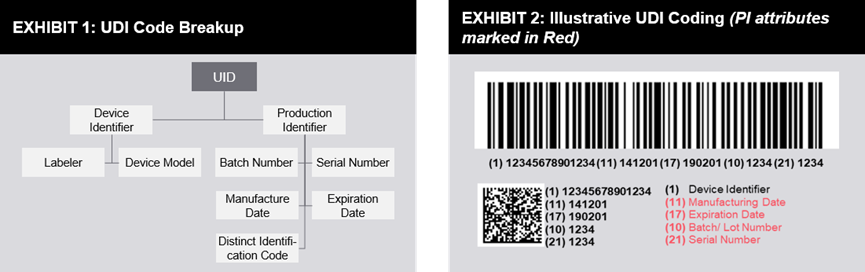
Mainly a code consisting of numbers or letters. It consists of a device identification code (DI) and a production identification code (PI).
The device identification code is a mandatory fixed code, which includes the information of the label management personnel, the specific version or model of the device, while the product identification code is not specially stipulated, and includes the device production batch number, serial number, production date, expiration date and management as a device. The unique identification code of the living cell tissue product.
Next, let's talk about GUDID, Global Unique Device Identification System (GUDID), FDA International Special Medical Device Identification Library. The database is made public through the AccessGUDID query system. Not only can you directly enter the DI code of the UDI in the label information on the database webpage to find the product information, but you can also search through the attributes of any medical device (such as the device identifier, company or trade name, generic name, or the model and version of the device). ), but it is worth noting that this database does not provide PI codes for devices.
That is, the definition of UDI: Unique Device Identification (UDI) is an identification given to a medical device throughout its life cycle, and it is the only "identity card" in the product supply chain. The global adoption of unified and standard UDI is beneficial to improve supply chain transparency and operational efficiency; it is beneficial to reduce operating costs; it is beneficial to realize information sharing and exchange; it is beneficial to monitoring adverse events and recalling defective products, improving the quality of medical services, and protecting patients safety.
Post time: Apr-28-2022

