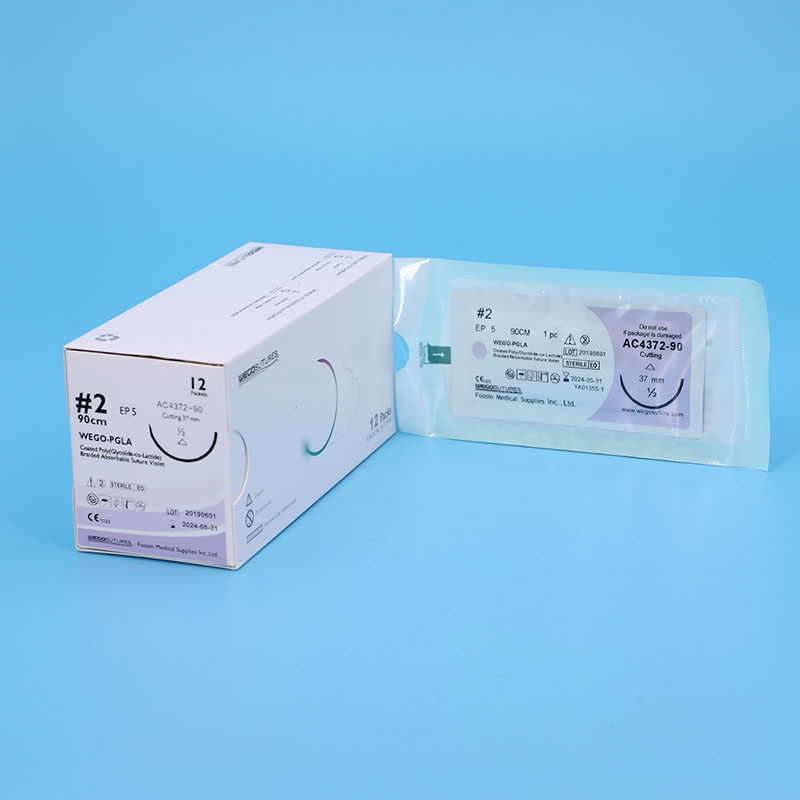
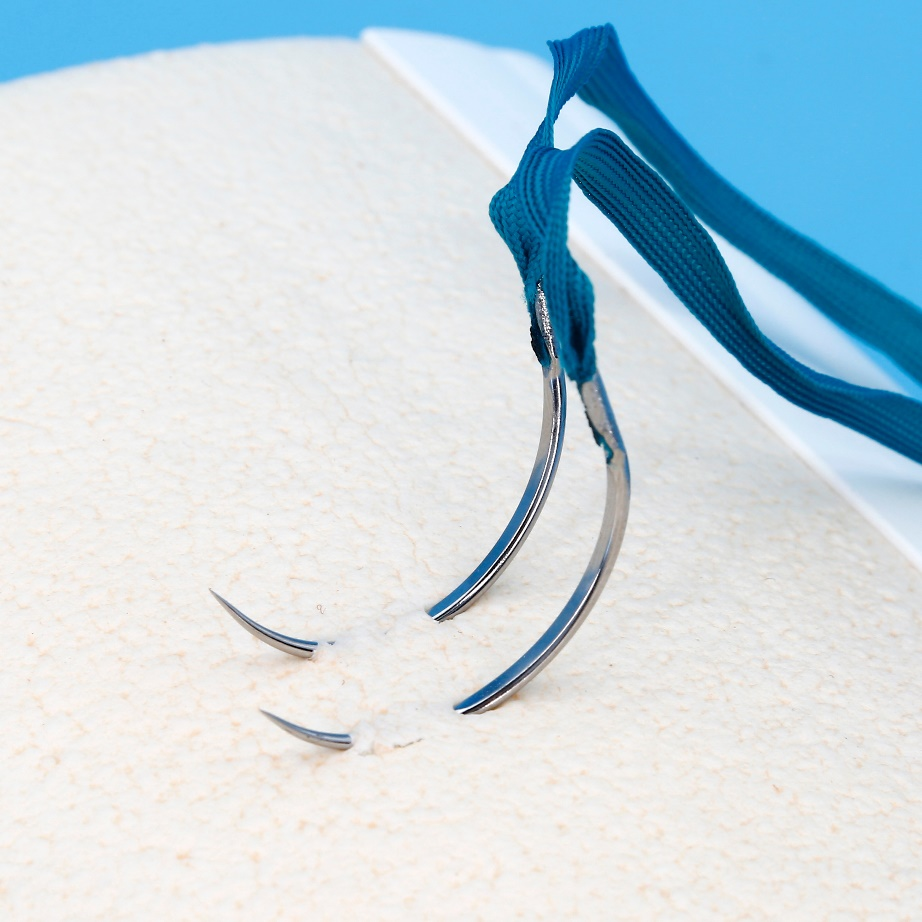
Sterile Multifilament Absoroable Polyglactin 910 Sutures With or Without Needle WEGO-PGLA
WEGO-PGLA suture
History of PGLA Suture
Polyglycolide Lactide (PGLA) absorbable surgical sutures, also called Polyglactin 910 per the percentage of components, developed to meet the requirement of safety absorption after implant, and to replace Catgut in the market. Since the Catgut thread was made by pieces of casing, which maintains the risk of weak tensile point in the whole thread that cannot offer consistence strength, and lower knot-pull strength than Synthetic sutures. And the higher tissue reaction rate compare with Synthetic materials also the reason why PGLA was developed. Even compare with PGA, the yarn of PGLA is softer and less stiffness base on the material characters since the 10% PLA makes the difference. Coated braided structure provides better knot security and smoothness than Catgut. Compare with Catgut it provides Predictable absorption by simple hydrolytic mechanism with greater strength. Vicryl is the first PGLA sutures in the market after the material developed by Johnson & Johnson.
Characteristic Features of WGO PGLA Suture
WEGO PGLA suture is coated by calcium stearate and 30:70 poly(glycolide-co-L-lactide) in order to create a smoother synthetic absorbable suture, combined by the special structure as the result of unique braiding technology, smoother than WEGO PGA, which brings easy tissue passage, and precise knot placement as compared with PGA suture.
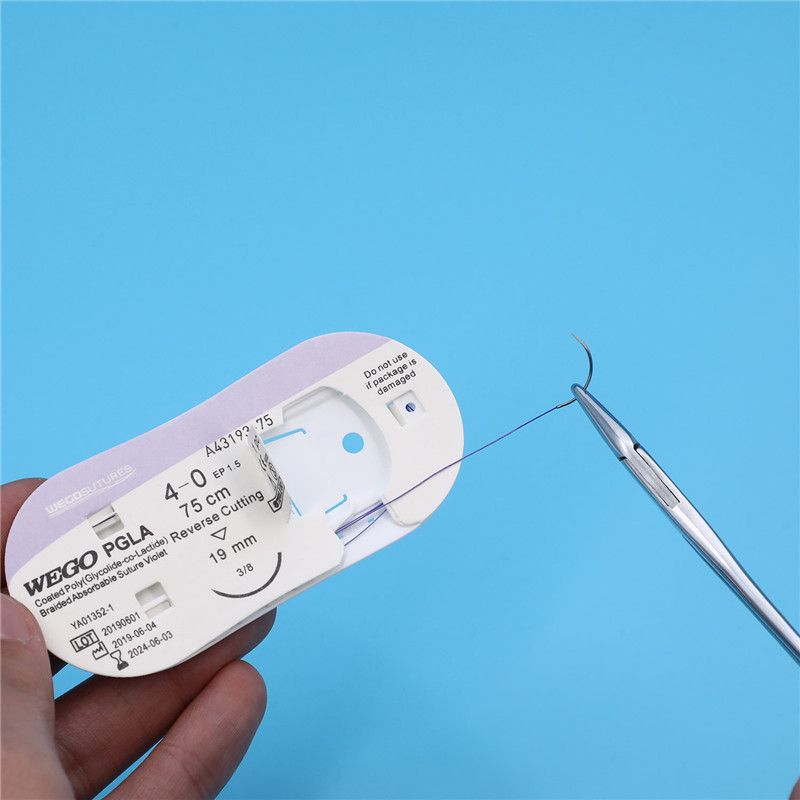
Armed by strong and sharp needle with atraumatic joint technology, WEGO PGLA brings easier healing performance.
A decreased tendency to irritate tissue.
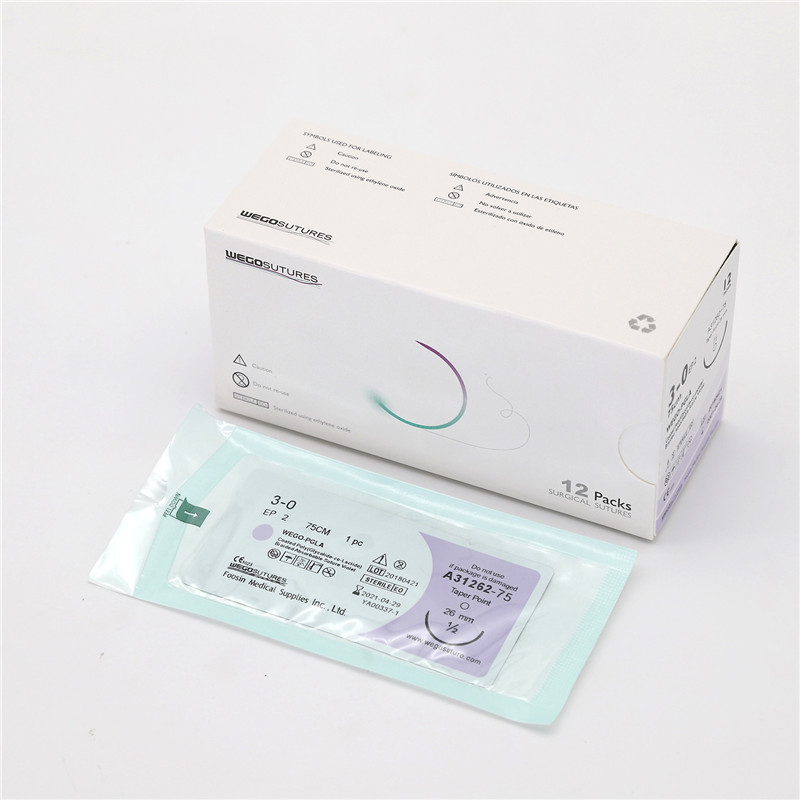
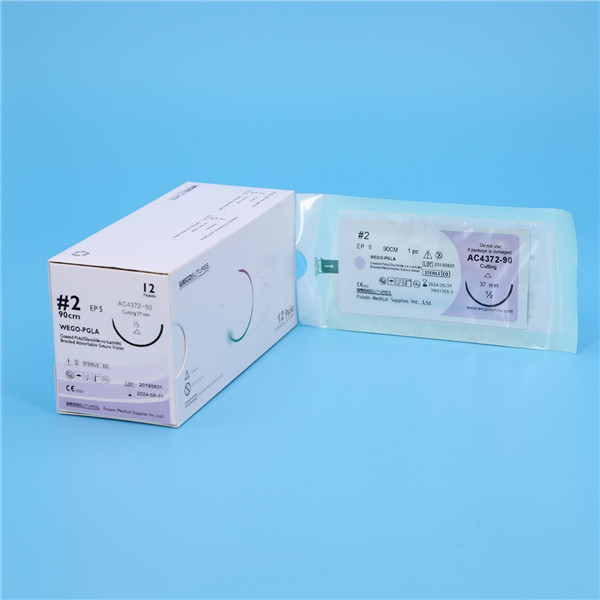
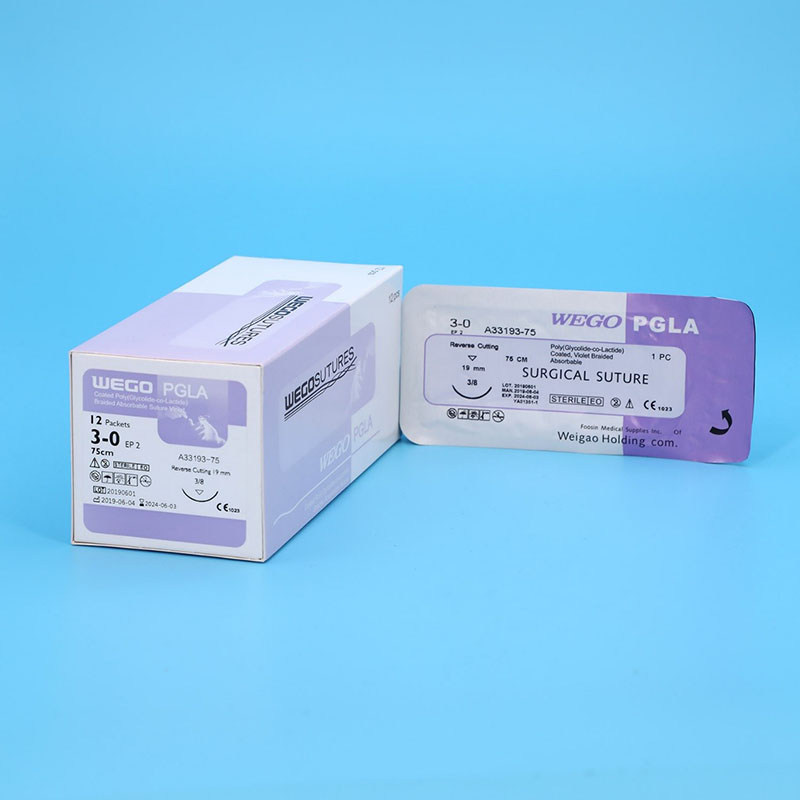
All WEGO PGLA sutures was fresh manufactured after order place to provide the longest shelf life to Client. Various Package from traditional aluminum foil with Figure 8 to Race-Tracy in Figure 0 with 12, 24 and 36 sutures per box design wrapped that can meet the requirement of end users.
OEM/ODM opening to global clients.
Indications of WEGO PGLA Suture
WEGO-PGLA uture is indicated for use in general surgery. It is suitable for the coating of soft tissue and for ligation, including use in ophthalmic procedures, but not for use in cardiovascular tissue and neual tissue. Applicable also in gynecology, pediatric surgery, gastrointestinal surgery and also in odontology. WEGO-PGLA suture is absorbable and should not be used where long suture support is necessary.
| WEGO-PGLA DATA SHEET | |
| Structure | Multifilament, braided |
| Chemical composition | Poly(glycolide-co-L-lactide) [Glacomer 91] |
| Coating | Poly(glycolide-co-lactide)(30/70)+Calcium Stearate |
| Color | Violet or Undyed |
| Sizes | USP 5- USP 8/0 metric 7 – metric 0.2 |
| Knot tensile strength retention | 7days post implantation 90% |
| 14days post implantation 75% | |
| 21days post implantation 50% | |
| 28days post implantation 20% | |
| Mass absorption | Degradation by hydrolysis within 56~70 days |
| Indications | General surgery, Gastroenterology, Urology, Plastic, Subcutaneous and intracutaneous closure, Gynaecology, Odontology, Paediatric surgery, Ophthalmology, Ligatures |
| Sterilization | Ethylene oxide |
| Certificate | CE,FDA,ISO13485 |
Packaging
WEGO-PGLA sutures are available sterile, as braided dyed (violet) and undyed (natural) in a variety of lengths and USP sizes, with or without needles.
WEGO-PGLA sutures already registered in China FDA and US FDA, ISO and CE certified. Registered in over 40 countries globally. The shelf life of WEGO-PGLA is 5 years. COA with all shipments